Read more:
New Delhi: The Central Drug Standard Control Organisation (CDSCO) in India has approved Eli Lilly’s tirzepatide, an active ingredient in the popular drugs Mounjaro and Zepbound. Last year, the US FDA approved Zepbound for treating obesity in adults.
All About Tirzepatide
The drug ,developed by Eli Lilly and Company, is primarily used to treat type 2 diabetes. It’s sold under the brand names Mounjaro for diabetes and Zepbound for weight loss. In India, it will be imported and marketed for diabetes treatment, with the obesity indication still under review. Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. It mimics these hormones to help regulate blood sugar levels.
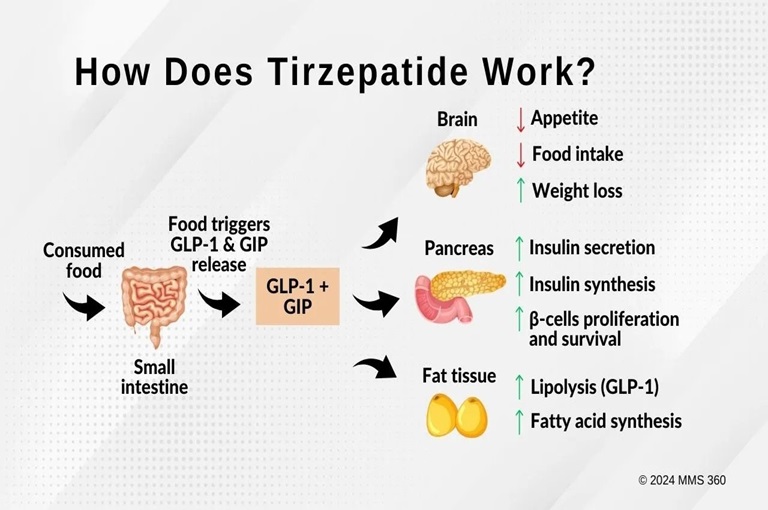
How Does Work?
Tirzepatide stimulates insulin release from the pancreas after meals, lowering blood sugar levels. It also reduces the release of glucagon, a hormone that increases blood sugar, thereby decreasing glucose production by the liver. Additionally, it slows stomach emptying, reducing the rate at which glucose enters the bloodstream after a meal. This helps control post-meal blood sugar spikes. Tirzepatide also acts on the brain’s appetite centers, reducing hunger and food intake, potentially aiding in weight loss.

Are There Any Side Effects?
While effective in managing type 2 diabetes, tirzepatide can have side effects. Common ones include nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, and abdominal pain. Serious side effects might include pancreatitis, low blood sugar if used with other medications, allergic reactions, severe stomach problems, vision changes, and gallbladder issues. It’s crucial to inform your healthcare provider if any side effects occur.
Precautions
- Usage: Your healthcare provider should demonstrate how to use Mounjaro before the first time.
- Low Blood Sugar: Discuss low blood sugar management with your provider.
- Birth Control: Oral birth control pills may be less effective with Mounjaro. Consult your provider.
- Regulatory Review: Eli Lilly’s CEO, David Ricks, mentioned that Mounjaro might launch in India by 2025 after regulatory review.
Tirzepatide represents a promising advancement in diabetes and weight loss management, offering significant benefits alongside manageable side effects.

FOLLOW FOR MORE .







